News
APP 1 Clip vs Push-On Style Baskets
Posted by Pam Bialiy on

The following article has been authored by John Heaney. Push-on style baskets have been used for dissolution testing for quite a few years at this point. While they’re generally accepted as equivalent to the clip style baskets pictured in USP <711>, it’s important to be aware of the differences between the two designs. Both have some advantages and disadvantages depending on the method. The clip style is officially recognized in <711>, so there is never any debate as to whether the clip style baskets are acceptable for testing. Furthermore, all the wetted materials are 316 Stainless Steel, which is chemically...
What is Intrinsic Dissolution and why is it important?
Posted by Pam Bialiy on

The following article has been authored by John Heaney. Intrinsic dissolution is a specific niche of dissolution testing often reserved for early R&D. The purpose is to provide scientists with an idea of the solubility of the API they’re studying. This data can be invaluable as it will help determine the excipients required to develop an oral dosage form. There are 2 types of methods for this. The first is a Rotating Disk (often called the Wood’s Apparatus) and the second is Stationary Disk. In both cases a pellet of the pure drug is pressed into a drug compact...
Why should I use a Cannula Stopper for Manual Sampling?
Posted by Pam Bialiy on

The following article has been authored by John Heaney. While automation is prevalent with dissolution testing, manual testing can and still should be done in certain circumstances. This can be when automation is too expensive, when the method is being developed and a certain amount of flexibility is needed, or when sample times are too short to be completed by automated systems. Manual sampling does offer significant flexibility but it also has some factors that need to be considered to ensure consistency. Sampling height is clearly defined in USP <711> as half-way between the top of the apparatus, be it...
Why should I use APP 3: Biodissolution?
Posted by Pam Bialiy on
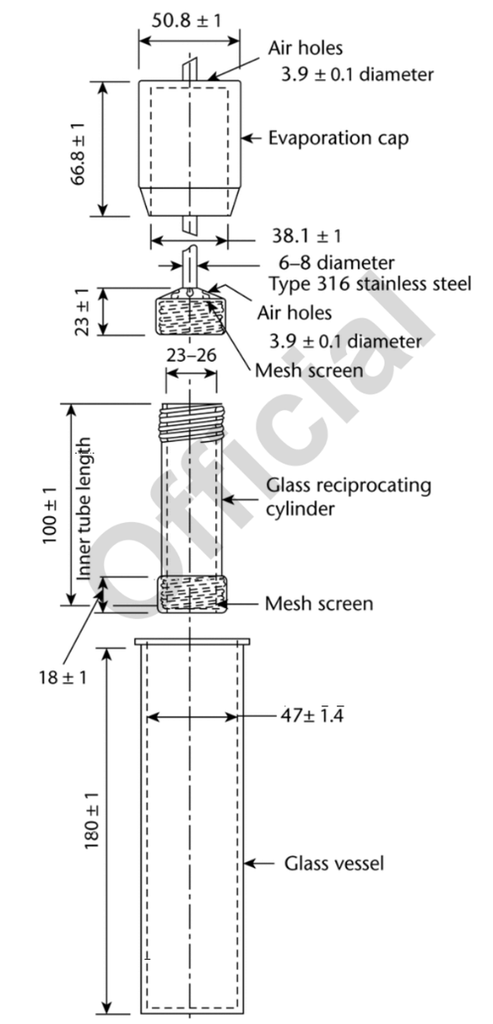
The following article has been authored by John Heaney. Compared to dissolution Apparatus 1 (Baskets) and Apparatus 2 (Paddles), Dissolution Apparatus 3: Biodissolution (the reciprocating cylinder) is quite rare. However, it has some key advantages over the more common baskets and paddles which can make it very useful for key applications. Apparatus 3 consists of a glass cylinder with a mesh screen at the bottom moving up and down in a specified distance and rate within a glass dissolution vessel. The dosage form is held within the glass cylinder. Vessels are typically arranged in rows allowing the glass cylinder...
Benefits of Sintered/Calendared vs Standard Mesh for APP 1
Posted by Pam Bialiy on

The following article has been authored by John Heaney. Calendared and sintered Stainless Steel (SS) mesh baskets are the norm for dissolution testing with good reason. This mesh provides several benefits to the basket that not just improves it but makes it usable in a lab in the first place. Calendared mesh is made by taking the SS wire mesh and passing the mesh between two rollers held at a specific distance from each other. The rollers press the high spots of the mesh, where the wires overlap, into the same height as the rest of the mesh. This results...
- Tags: APP 1, Apparatus 1, basket method, Calendared, Dissolution Baskets, Mesh, Sintering, SS Mesh
