News — dissolution testing
What is Intrinsic Dissolution and why is it important?
Posted by Pam Bialiy on

The following article has been authored by John Heaney. Intrinsic dissolution is a specific niche of dissolution testing often reserved for early R&D. The purpose is to provide scientists with an idea of the solubility of the API they’re studying. This data can be invaluable as it will help determine the excipients required to develop an oral dosage form. There are 2 types of methods for this. The first is a Rotating Disk (often called the Wood’s Apparatus) and the second is Stationary Disk. In both cases a pellet of the pure drug is pressed into a drug compact...
Why should I use APP 3: Biodissolution?
Posted by Pam Bialiy on
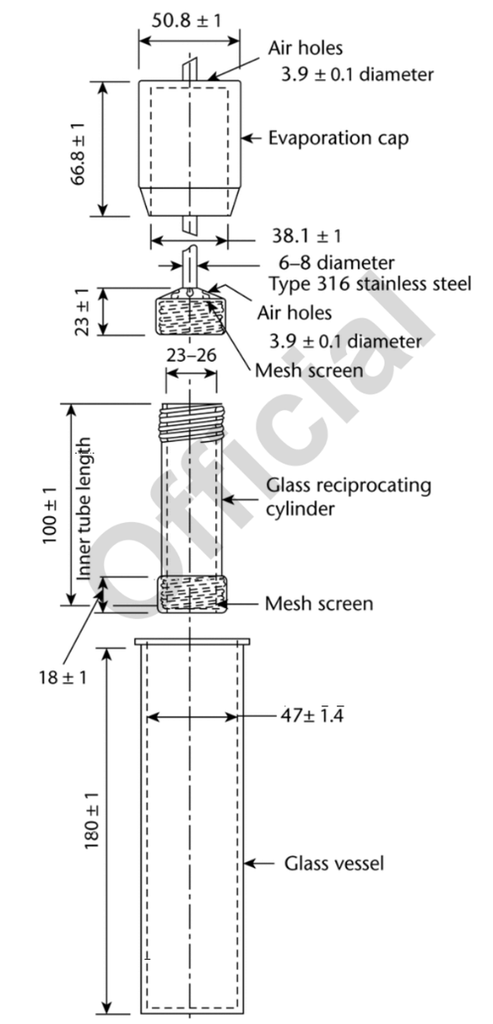
The following article has been authored by John Heaney. Compared to dissolution Apparatus 1 (Baskets) and Apparatus 2 (Paddles), Dissolution Apparatus 3: Biodissolution (the reciprocating cylinder) is quite rare. However, it has some key advantages over the more common baskets and paddles which can make it very useful for key applications. Apparatus 3 consists of a glass cylinder with a mesh screen at the bottom moving up and down in a specified distance and rate within a glass dissolution vessel. The dosage form is held within the glass cylinder. Vessels are typically arranged in rows allowing the glass cylinder...
Impact of Deaeration on Dissolution
Posted by Pam Bialiy on
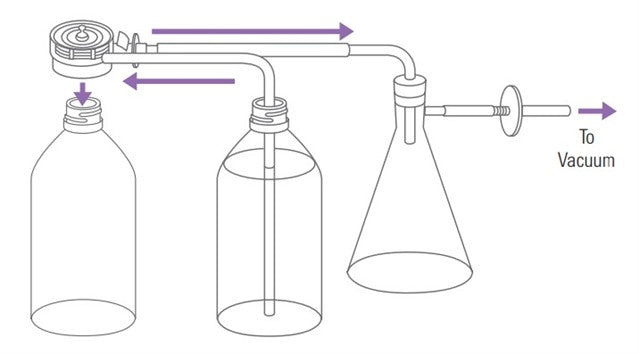
The following article has been authored by John Heaney. Deaeration, or degassing, of dissolution media is not required for every dissolution method, but when it is required it is critically important. The USP Prednisone PVT requires deaeration of the dissolution media and the tablet is formulated to show whether or not the deaeration method is adequate. Deaeration tends to have a larger impact on dissolution results as compared to other factors regarding the dissolution test. How this shows itself can change depending both on the apparatus, and the dosage being tested. How to deaerate is largely up to the...
Hydrodynamic Effects of Resident vs Manual Probes/Cannula
Posted by Pam Bialiy on

The following article has been authored by John Heaney. Does the presence of a resident sampling probe affect the hydrodynamics of a dissolution vessel? Yes, yes it does. The more important questions are how strong is the change in the hydrodynamics and is it strong enough to affect testing? USP <1092>, an advisory chapter addresses this directly with: Sampling probes may or may not remain in the vessel throughout the entire run. Sampling probes or fiber-optic probes can disturb the hydrodynamics of the vessel; therefore, adequate validation should be performed to ensure that the probes are not causing a significant...
Why use Apex Vessels?
Posted by Pam Bialiy on

The following article has been authored by John Heaney. Coning of disintegrating dosage forms is fairly common when using Apparatus 2 (Paddles) to perform dissolution testing. The reason for this is the spinning of the paddle causes a cone shaped zone to form directly beneath it where there is low hydrodynamic activity. In other words, it’s a bit of a dead zone where the stirring has little effect, and the particles are allowed to settle. Depending on the formulation this coning can be problematic. It can prevent drug from dissolving in the media due to a reduction in the effective...
